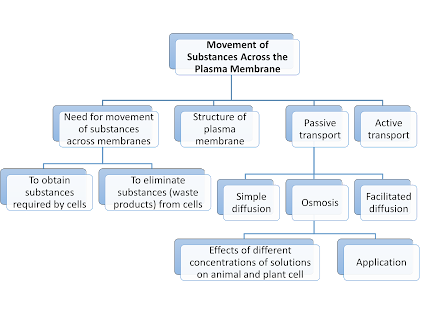
The movement of substances across a plasma membrane occurs through Passive Transport and Active Transport.
Passive Transport
Definition: The movement of substances across the plasma membrane from a region of high concentration to a region of low concentration (down the concentration gradient).
Characteristics:
1. Do not require energy.
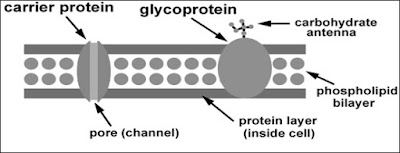
2. Substances move across the plasma membrane through:
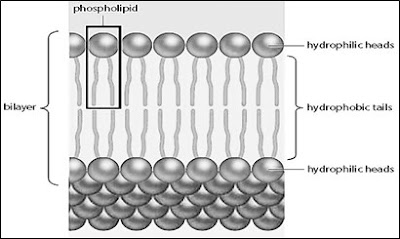
- Phospholipid bilayer
- Channel protein
- Carrier protein
3. Three ways of passive transport:
- Simple diffusion
- Osmosis
- Facilitated diffusion
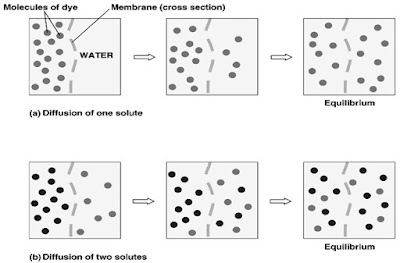
Simple diffusion
1. Definition = the movement of molecules down the concentration gradient until equilibrium is reached.
2. The molecules are evenly distributed with uniform concentration.
3. The bigger the concentration gradient, the faster the rate of diffusion.
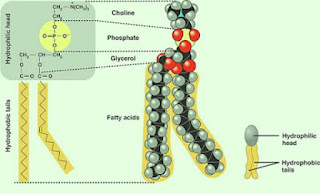
4. Soluble substances that can move through phospholipid bilayer as simple diffusion:
- Small uncharged polar (water soluble) (e.g. oxygen, carbon dioxide, water)
- Lipid soluble molecules (e.g. fatty acids, glycerol, vitamins A,D,E,K)
 |
| Simple diffusion |
5. Examples of simple diffusion:- Gases exchange at alveolus and blood capillary
- Gases exchange between body cell and blood capillary
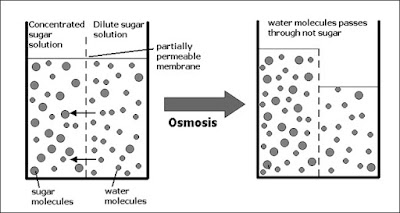
Osmosis (the passive transport of WATER)
1. Definition = the diffusion of water molecules down their concentration gradient through a semi-permeable membrane (selectively permeable membrane).
2. Water molecules move from a region of higher water concentration to region of lower water concentration.
3. Examples: The absorption of water by root hairs of a plant.
 |
| Osmosis |
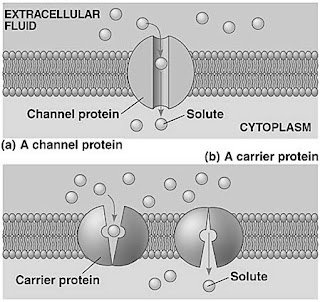
Facilitated diffusion
1. Definition = the passive transport of substances across the phospholipid bilayer with the help of transport proteins (channel protein & carrier protein).
2. The rate of facilitated diffusion depends on:
- The number of transport protein molecules in the membrane.
- How fast they can move their specific solute.
3. Substances move through facilitated diffusion:
- Channel protein: Small charged molecules (e.g. mineral ions).
- Carrier protein: Uncharged polar molecules (molecules insoluble in fats) (e.g. glucose, amino acid).
4. Mechanism of carrier protein:
The solute moves to the specific binding site of the carrier protein.
▼
The solute binds to the carrier protein at the binding site & triggers the carrier protein to change its shape.
▼
Carrier protein changes its shape and moves the solute across the membrane.
▼
The carrier protein returns back to its original shape.
 |
| Facilitated diffusion |
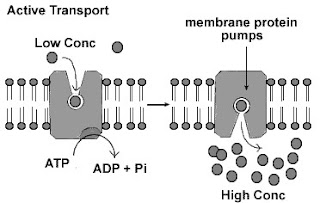
Active
Transport
Definition: the movement of substances across the plasma membrane from a region of low concentration to a region of high concentration (against the concentration gradient).
- Require
Energy (ATP = Adenosine Triphosphate)
- The transport protein use
energy to change the shape of the protein & to pump or transport the
substance across the membrane
- Examples:
- pumping of sodium ions
(Na+) out of the cell
- intake of mineral ions by the root hairs
of a plant
 |
| Active Transport |
Comparison
between Passive Transport & Active Transport
 |
Comparison
between Passive Transport & Active Transport |