Carbohydrate is an organic compound containing carbon, hydrogen and oxygen elements in a 1:2:1 ratio. Cx(H2O)y is the typical formula for carbohydrate. Among the roles of carbohydrate are:
- Storage of food.
- As a source of energy.
- Structural components of organisms.
- Components of polymers, for example, nucleic acid.
- Defense and protection.
- Provides mechanical support.
There are 3 main groups of carbohydrates, namely:
- Monosaccharide
- Disaccharide
- Polysaccharide
Monosaccharides
1. Monosaccharides also known as simple sugars. They are named with the suffix -ose.
2. Monosaccharides contain either an aldehyde group (-CHO) or ketone group (C=O).
3. Monosaccharide with an aldehyde group is called aldo-sugars or aldose; while the other is referred as keto-sugar or ketose.
4. The general formula for monosaccharide is (CH2O)n. If n=3, the sugar is called triose; n=5, a pentose sugar; and n=6, a hexone sugar.
5. Physical properties:
- Sweet.
- The molecule is small with a low molecule mass compared to other sugars.
- Can crystallize.
- Soluble in water.
6. Chemical properties:
- Can reduce Benedict's solution and Fehling's solution due to the presence of free aldehyde or ketone group in molecule.
- Can undergo condensation reactions to form disaccharides or polysaccharides.
7. General functions of monosaccharides:
- Provide energy.
- As monomer for polysaccharides and disaccharides.
- Pentose is an important component of nucleic acid.
- Monosaccharides are soluble in water. Most carbohydrates are transported in the form of monosaccharides.
Triose
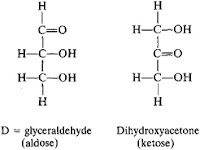 |
| Glyceraldehydes and dihydroxyacetone |
1. Molecule formula: C3H6O3
2. Example:
- Glyceraldehydes
- Dihydroxyacetone
3. Functions of triose:
- Triose is an important intermediate in Krebs Cycle (respiration).
- Triose is used to synthesize starch and glucose in photosynthesis.
Pentose
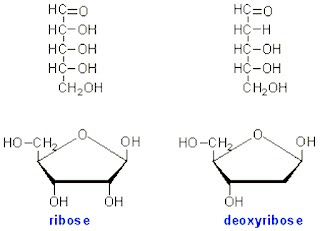 |
| ribose deoxyribose |
1. Molecule formula: C5H10O5
2. Examples:
- Ribose
- Deoxyribose
- Arabinose
- Xylose
- Ribulose
- Xyllulose
3. Functions of pentose:
- Ribose and deoxyribose are main components of nucleic acid (DNA and RNA).
- Ribose is a component of ATP molecule.
- NAD (nicotinamide adenine dinucleotide), FAD (flavin adenine dinucleotide) [Both the FAD and NAD are electron carriers] and coenzyme A are synthesized using pentose.
Hexose
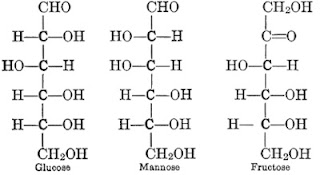 |
| Hexose |
1. Molecule formula: C6H12O6
2. Examples:
- Glucose
- Manose
- Galactose
- Fructose
- Sorbose
3. Most glucose molecules exist in the ring form or cyclic form. The two common isomers or glucose are α-glucose and β-glucose.
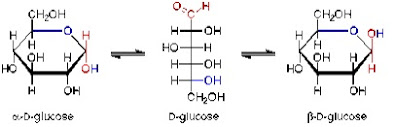 |
| Different forms of glucose |
4. Fructose occurs as straight-chain or linear molecules or in a ring form.
5. Functions of hexose:
- Glucose is the main substrate for respiration.
- Hexose is the monomer for disaccharides and polysaccharides.
- Most carbohydrates are transported as hexose in the blood stream.
Chemical tests for monosaccharides
1. All monosaccharides are reducing sugar. This is due to the presence of free aldehyde or ketone group in molecule.
2. When monosaccharides are 🔥🔥heated🔥🔥 with Benedict's solution or Fehling's solution, a brick red precipitation is formed. The brick red precipitation is Copper (I) Oxide, Cu₂O.
No comments:
Post a Comment