Water
Water is the main constituent of organisms and is also a medium for organisms to live. The unique physical and chemical properties of water enable water to carry out these its roles.
Physical properties of water:
- Water is transparent and colorless.
- Water melt at 0°C and boils at 100°C. Thus, water is a liquid at room temperature.
- Water is a universal solvent. It dissolves more substances than any other solvent.
- High surface tension.
- Very high specific heat capacity. The specific heat capacity of water is 4184 J g-1 K-1. The specific heat capacity for water is among the highest for any known material.
- High latent heat of vaporization.
- High latent heat of fusion.
- Density of ice is lower than liquid water.
- Low viscosity.
Chemical properties of water:
- Water takes part in hydrolysis reaction.
- Water can undergo photolysis (during photosynthesis).
Water molecule's structure:
- Water molecule has a "V" shape and is a polar molecule.
- The oxygen atom has a partial negative charge while the hydrogen atoms have partial positive charge.
- 1 single water molecule can be bonded to 4 other water molecules by hydrogen bonds.
- At 37°C, about 15% of water molecules are bonded to each other by hydrogen bonds.
- In ice, all the water molecules bonded by hydrogen bonds; hence the ice have a slightly larger volume than the water forming it.
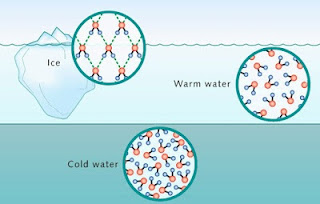 |
| Comparison between solid water (ice) and liquid water molecules |
Important properties of water as a medium for life:
- Water is transparent and colorless. These properties enable aquatic animals to see their surroundings.
- Water has high surface tension. This enable aquatic insects (mosquito larvae, water beetles, and water scorpions) to stick below the surface of water to breath.
- Water is a liquid at room temperature. This provides a liquid medium aquatic organisms to live and move.
- Water has a very high specific heat capacity. Thus aquatic habitats have relatively stable temperature.
- Density of ice is lower then liquid water. Hence the aquatic habitats start freezing from the surface. The layer of ice on the surface is an effective heat insulator. So only the surface of aquatic habitats freezes during winter.
- Water has low viscosity. This enable aquatic animals to move with ease in water.
- Water has high latent heat of fusion. Thus aquatic habitats are slow to freeze. A high latent heat of fusion means that a lot of heat has to be expelled from the water at 0°C before it could freeze (solidify).
Important properties of water as a constituent for life:
- Water is a universal solvent. This allow many substances to dissolve and react inside the cells. Chemical reactions only occur in aqueous solution.
- Water has a very high specific heat capacity. Thus the temperature inside organisms homeotherms is relatively stable.
- Water has high specific heat of fusion. Thus is content of cell is slow to freeze.
- Water has high latent heat of vaporization. Thus sweating is a very effective cooling mechanism for mammals. This enable mammals to occupy a wider range of habitats.
- Water has low viscosity. This enable materials to be transported quickly in organisms.

Finally, you’re back ! I’m so glad for this 🥺❤️ THANK YOU SO MUCH !! I hope you’ll never stop updating it.
ReplyDeleteHi. Thank you for supporting me and now I am motivated💪😊 I will appreciate if you can share my blogs to your juniors or seniors that you think he or she may need these as their references. Last but not least, thank you and stay healthy😊
DeleteHi. Thank you for supporting me and now I am motivated💪😊 I will appreciate if you can share my blogs to your juniors or seniors that you think he or she may need these as their references. Last but not least, thank you and stay healthy😊
DeleteFinally, you’re back ! I’m so glad for this 🥺❤️ THANK YOU SO MUCH !! I hope you’ll never stop updating it.
ReplyDelete