 |
| SPM Biology 4 Mind Map |
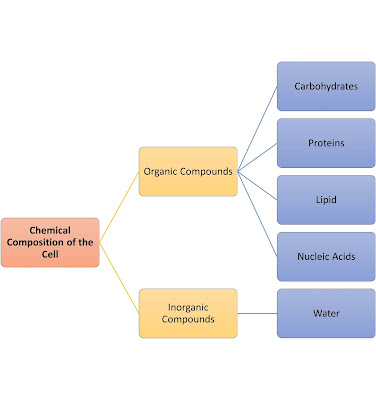
Properties of water and its importance in a cell
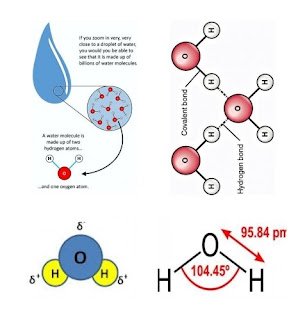
1. Polarity of water
- Water is inorganic compound.
- Consist of hydrogen and oxygen elements.
- Polar molecules - produce hydrogen bonds and allow water to act as universal solvent.
- Allow solutes such as glucose to be transported into cells.
2. Cohesive force and adhesive force of water
- Cohesive force: Water molecules are attached to each other.
- Adhesive force: Water molecules are attached to other surfaces.
- Both forces produce capillary action (allow water to move along narrow spaces, eg. xylem tube).
3. Specific heat capacity of water
- Water has a high specific heat capacity (4.2kJ/kg/°C) - 4.2kJ of heat energy required to raise the temperature of one kg of water by 1°C.
- Water absorbs a lot of heat energy with a small rise in temperature. This helps to maintain the body temperature of organisms.
No comments:
Post a Comment