1. Distribution:
- Cellulose is the most common organic materials. It is found in all plant cells. 20% to 50% of the cell wall of plants is cellulose.
- Also found in cotton (90% cellulose).
2. Physical properties:
- Not soluble in water.
- Cannot crystallize.
- Not sweet.
- High relative molecular mass.
3. Chemical properties:
- Can undergo hydrolysis to form β-glucose.
- Reacts with iodine solution to form brownish-yellow complex.
Structure of Cellulose
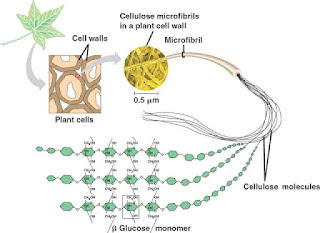
1. Cellulose has long unbranched chain molecules. Cellulose molecule consists of β-glucose molecules linked by 1,4-glycosidic bonds.
2. Neighboring cellulose molecules are cross-linked by hydrogen bonds.
3. Cross-linking between neighboring chains by hydrogen bonds produces a strong structure.
4. In cell walls, numerous cellulose molecules (about 2,000) are cross-linked to form cellulose microfibrils. Many microfibrils are bound together to form fibrils that form the cell walls.
 |
| Cellulose |
Functions and properties of cellulose related to its function
1. Cellulose is source of food and energy for herbivorous animals, bacteria and fungi. It is because it can undergo hydrolysis to form glucose. Glucose is the main substrate for respiration.
2. Cellulose are structural materials for plants. It is due to:
- Cellulose have long straight-chain molecules.
- Cellulose is not soluble in water.
- Hydrogen bonds between neighboring molecules produce a strong structure.
Structural differences between cellulose and starch
- Cellulose molecules consist of β-glucose monomers; while the starch molecules consist of α-glucose monomers.
- Cellulose molecules are long straight-chain molecules; while starch molecules are coiled into helix and having branched chain.
- Hydrogen bond occurs between neighboring cellulose molecules. However there are no hydrogen bonding between neighboring starch molecules.
No comments:
Post a Comment